CHEN2000 Process Principles
GROUP PROJECT Semester 2, 2021
本文是excel代写的完美范例:工艺原理作业代写 The problems related to submission 3 take into account many of the answers to the previous problems (in Parts 1 and 2) and relate
INTRODUCTION
The group project is designed to help you learn the lecture content as you go along from week to week. The project comprises of three parts (with weight percentages of 30, 35 and 35 towards the final project mark) such that you can commence with tasks following the completion of relevant lectures and workshops each week.
You may need to do a bit of your own research, and also learn how to work effectively in a team. Good teamwork is often more than the sum of individual contributions.
PROCESS DESCRIPTION 工艺原理作业代写
A series of problems including energy shortage and green-house gas emissions are arising as a consequence of huge fossil fuel consumption. The intergovernmental Panel on Climate Change (IPCC) has emphasized the adoption of bio-energy and carbon capture and storage (BECCS) as a leading technology towards meeting the global warming target in their Fifth Assessment Report (AR5). The attractiveness of BECCS is related to its negative emission potential, which if implemented on a large scale, can compensate for the emissions dismissed for a mid-century, and might go further towards achieving a negative account balance after 2050 (Ghiat et al., 2020).
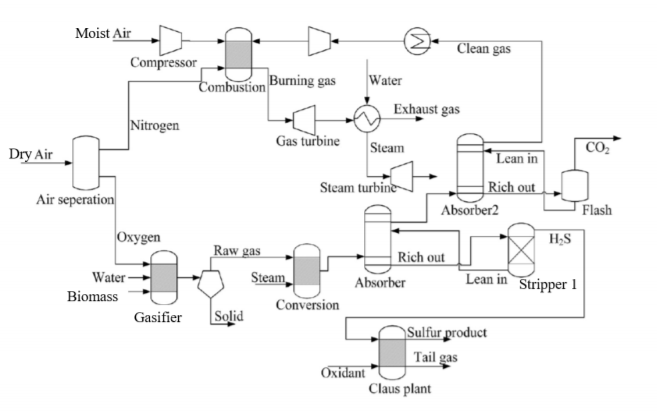
You are assigned to develop a biomass-based integrated gasification combined cycle (IGCC) with post-combustion CO2 recovery by Selexol absorption process. Which could improve the efficiency of power generation with less impact on the environment. An overall schematic of the biomass based integrated gasification combined cycle is illustrated in Figure 1. The integrated system consists of three main sections: the first consisting of the biomass gasification process, and the second entails the acidic gas removal process. Municipal solid wastes are utilized as biomass feed to the system. The quantitative analysis has been determined by the proximate and ultimate analyses as illustrated in the given group data.
As illustrated in Figure 1, biomass is first fed to the gasifier to convert the non-conventional feed to its elemental composition that include carbon, hydrogen, oxygen, nitrogen and sulfur.
The syngas product of this process is then transferred to a water shift reactor to convert CH4 and CO into clean hydrogen fuel. To control the emission of acidic gas to environment, two stages Selexol absorption processes are designed to provide clean gas with minimum hazardous gases of H2S and CO2. Recovered H2S will be further converted to elemental sulfur product for other application. Apart from that, CO2 gases will be captured and regenerated from flash tank before storing it in liquid form.
As the result, clean gas will be channelled to combustion chamber together with new feedin of fresh air. After that, hot burning gas will be used to turn the gas turbine and generate steam for power generation.

DATA 工艺原理作业代写
The biomass composition and gasification products required for this plant are specified for your group, as a separate file on the Blackboard (Data for Group Project S2 2021.pdf).
PROBLEMS: Part 1
- Based on the data given to your group, find the mole composition of biomass. [5 marks]
- Draw the boundary of system (with dotted line) involving biomass gasification, water-shift reaction, acidic gas removal and combustion reaction on Figure 1. [5 marks]
- Assuming all carbon elements had been fully reacted, what is the ratio of selectivity for the products of CH4 and CO. [5 marks]
- Determine the limiting reactant in the water-shift reaction and yield of hydrogen. Also, find the outlet composition (exhaust gas) of water-shift reactor by performing a complete component mass balance and validate the results with data from element mass balance.
Hint: Fixed H, N, O and S element in biomass will be converted to H2, N2, O2 and H2S respectively. [35 marks]
- What are the advantages and disadvantages of biomass-based integrated gasification combined cycle (IGCC) in comparison to a conventional coal-based power plant? To ensure the quality of fuel (clean gas) before entering combustion chamber for power generation, acidic gases such as CO2 and H2S must be treated with Selexol (Poly(ethylene glycol) dimethyl ether-DMPEG) absorption process. Calculate the amount of Selexol needed to achieve the standard of clean gas set in the data given for each group. Find the quality of the clean gas channelling to combustion chamber.
Hint: Perform a complete mass balance for the whole absorption process [30 marks]
INSTRUCTIONS (Part 1)
The link for each submission will be available a week before the due date. Each part of the
report should include:
• An Excel spreadsheet with detailed workings
- Please use the template provided on the Blackboard and detail your calculations on the same worksheet.
• A Word/PDF document:
- a Title page including the project title, unit name and names of team members
- assumptions and basis for each calculation, and sample calculation if necessary
- key equations used to solve the problem
- references
There should only be 1 Word/PDF document and 1 Excel submission for each group. It is your collective responsibility to ensure that your group member(s) have submitted both these before the deadline. Please submit the Word/PDF document to the Turnitin link on the Blackboard and submit the Excel spreadsheet to the Excel submission link on the Blackboard.
Label each file as follows:
Group #_ Part 1.xlsx
Group#_Part1.pdf OR Group#_Part1.docx
Please refer to Curtin Student Plagiarism guidelines to ensure your submission meets them. Please do not collude with any other group/person in completing this project.
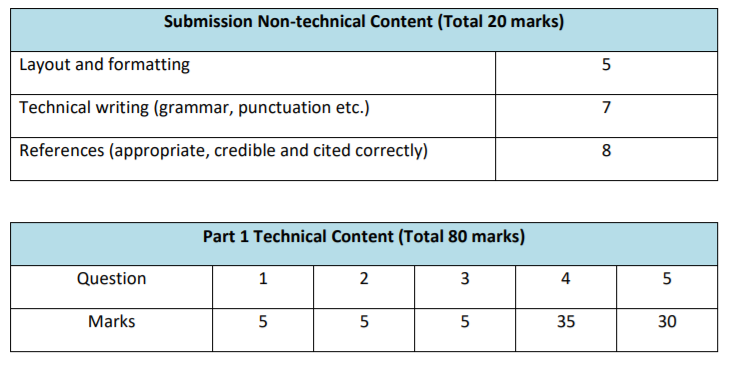
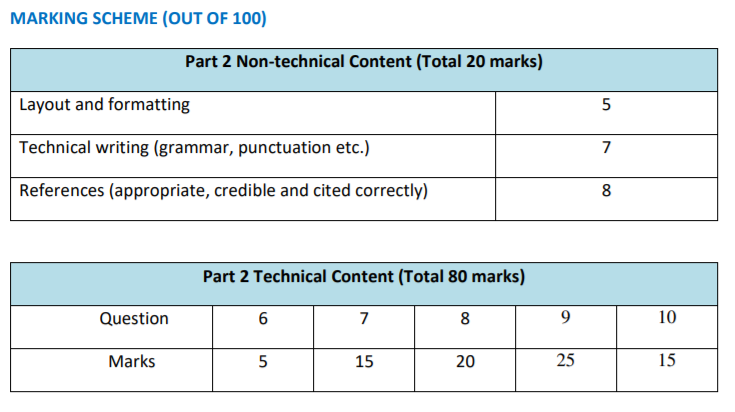
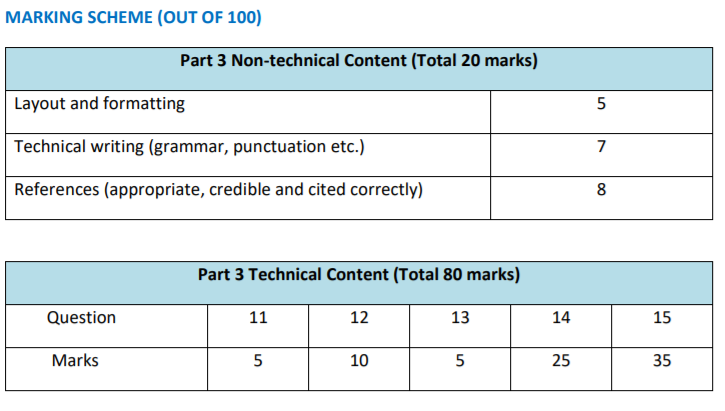
MARKING SCHEME (OUT OF 100)
PROBLEMS: Part 2 工艺原理作业代写
- Calculate amount of excess air supply to the combustion chamber in mole percentage. Assuming all fuels undergo complete combustion. [5 marks]
- Consider the combustion chamber C: 1 kg of clean gas is burnt with 10% excess air (containing 1.5 mol% moisture) to generate heat as it is an exothermic reaction.
Combustion reactions can have multiple side reactions and often there are many reasons for not going to completion, or forming the products expected.
Assuming you do not know what reactions are taking place inside the combustion chamber, and assuming you do not know if all reactions have gone to completion, perform a Degree of Freedom analysis for the following possible combinations of exhaust gas:
i. Only CO2, H2O, O2 and N2 are in the exhaust
ii. Only CO, CO2, H2O, O2 and N2 are in the exhaust
iii. Only H2, CO, CO2, H2O, SO2, O2 and N2 are in the exhaust
You do NOT have to write or solve any equations, but simply use the element balance to state clearly NU and NE (and what they are), and therefore calculate ND for each case. [15 marks] 工艺原理作业代写
- Assuming it is a complete combustion. Calculate the exhaust gas composition (flue gas) in mol% and mass%, if clean gas is burnt with 10% excess air (with 1.5 mol% moisture), to produce the composition specified in Q7 (i). Tabulate the exhaust gas composition in the form of Orsat analysis. [20 marks]
Assuming it is an incomplete combustion. Calculate the exhaust gas composition (flue gas) in mol% and mass%, if clean gas is burnt with 10% excess air (with 1.5 mol% moisture), to produce the composition specified in Q7 (iii). Assume you have 0.8 mol% of your wet exhaust gas at unburnt CH4 and the CO/CO2 ratio in the wet exhaust gas is 1/10.
Hint: Use the excel solver function with your explicit equations, and consider how many decimal places you need to consider when calculating values. [25 marks]
- By channelling all CO2 gaseous to the absorber unit 2 and flash out the pure CO2 for storage. Determine the outlet’s pressure and temperature of flash column to ensure CO2 gases can be stored in liquid form. Sketch a p-T and P-V diagram for CO2 showing the saturation pressure at your calculated dewpoint temperature. If we wanted the dewpoint to be 25 °C, what pressure of CO2 would the CO2 need to have? State your assumptions. [15 marks]
INSTRUCTIONS (Part 2)
The link for each submission will be available a week before the due date. The report should include:
• An Excel spreadsheet with detailed workings
- Please use the template provided on the Blackboard, and detail your calculations on the same worksheet.
• A Word/PDF document:
- a Title page including the project title, unit name and names of team members
- assumptions and basis for each calculation, and sample calculation if necessary
- key equations used to solve the problem
- references
There should only be 1 Word/PDF document and 1 Excel submission for each group. It is your collective responsibility to ensure that your group member(s) have submitted both these before the deadline. Please submit the Word/PDF document to the Turnitin link on the Blackboard and submit the Excel spreadsheet to the Excel submission link on the Blackboard.
Label each file as follows:
Group #_ Part2.xlsx
Group#_Part2.pdf OR Group#_Part2.docx
Please refer to Curtin Student Plagiarism guidelines to ensure your submission meets them.
Please do not collude with any other group/person in completing this project.
PROBLEMS: Part 3
The problems related to submission 3 take into account many of the answers to the previous problems (in Parts 1 and 2) and relate to your original design of the outdoor shower.
- Stating your assumptions, calculate the volumetric flow rate (m3 /hr) of your clean gas for 100 mol/hr entering at 25 °C and 1 atm. [5 marks]
What is the dewpoint of the moist air? Sketch a p-T and P-V diagram for H2O showing the saturation pressure at your calculated dewpoint temperature. If we wanted them dewpoint to be 20 °C, what mole fraction of water would the air need to have so that it can easily be removed before entering compressor due to cavitation problem? State your assumptions. [10 marks]
Write a general energy balance for the combustion chamber, using notation for symbols used in lectures. Stating your assumptions, show that the heat added/removed Q is equal to the total heat of reaction ΔH°reaction. [This is a theoretical question and does not need any number working.] [5 marks]
Using balanced equations for each of your syngas reactions, calculate the standard heat of reaction ΔH°rxn,298K per mole of clean gas for each of the 4 reactions in your fuel, using either heats of formation data or heats of combustion data. What is the standard heat of reaction ΔH°rxn,298K for your clean gas, in kJ/kg?
Hint: Using the same fractional composition found in Q5 (Clean gas composition). [25 marks]
- Assuming it is an adiabatic reaction inside the combustion chamber. Calculate the temperature of your exhaust gas. How many mol/hr of clean gas is required to provide the heat required to heat the exhaust gas to 2450oC for power generation. State all your assumptions. [35 marks]
INSTRUCTIONS (Part 3)
The link for each submission will be available a week before the due date. The report should include:
• An Excel spreadsheet with detailed workings
- Please use the template provided on the Blackboard, and detail your calculations on the same worksheet.
• A Word/PDF document:
- a Title page including the project title, unit name and names of team members
- assumptions and basis for each calculation, and sample calculation if necessary
- key equations used to solve the problem
- references
There should only be 1 Word/PDF document and 1 Excel submission for each group. It is your collective responsibility to ensure that your group member(s) have submitted both these before the deadline. Please submit the Word/PDF document to the Turnitin link on the Blackboard and submit the Excel spreadsheet to the Excel submission link on the Blackboard.
Label each file as follows: 工艺原理作业代写
Group #_ Part3.xlsx
Group#_Part3.pdf OR Group#_Part3.docx
Please refer to Curtin Student Plagiarism guidelines to ensure your submission meets them.
Please do not collude with any other group/person in completing this project.
References
Ghiat, I., AlNouss, A., McKay, G., & Al-Ansari, T. (2020). Biomass-based integrated gasification combined cycle with post-combustion CO2 recovery by potassium carbonate: techno-economic and environmental analysis. Computers & Chemical Engineering, 135, 106758.
Ren, S., Feng, X., & Wang, Y. (2021). Emergy evaluation of the integrated gasification combined cycle power generation systems with a carbon capture system. Renewable and Sustainable Energy Reviews, 147, 111208.
Khonde, R., Hedaoo, S., & Deshmukh, S. (2021). Prediction of product gas composition from biomass gasification by the method of Gibbs free energy minimization. Energy Sources, Part A: Recovery, Utilization, and
Environmental Effects, 43(3), 371-380.

更多代写: HomeWork cs作业 金融代考 postgreSQL代写 IT assignment代写 统计代写 英国论文代写机构




发表回复
要发表评论,您必须先登录。