CHEM-E7100, Engineering Thermodynamics, Separation Processes, part 1
工程热力学代写 The model electrolytes are KCl – LiCl and KCl – HCl whose SLE data are shown in the following tables reported in literature at 25°C
Homework Assignment 1, 2022
HW1 HW ID # ___________
student ID # __________ This HW1 has two independent systems, non-electrolyte and electrolyte
Learning objectives: 工程热力学代写
Databases, evaluation of experimental data, pure component properties, phase equilibria of binary systems, getting familiar with Aspen
Upload your documents into MyCourses. To check many home works efficiently I ask to name the files like this:
ETSP_p1_HWx_familyname_givenname_inputY.ext
HWx = HW1 (home work 1) or HW2 (home work 2)
familyname_givenname
inputY, where Y = 1, 2, 3, … (1st, 2nd, 3rd,.. upload)
ext = docx, xlsx, pdf, apw, … . (this is file extension, i.e. file type)
Docx file is the master document, xls or xlsx or apwz files are supporting information.
Student dependent values can be read from a separate file once the enrollment is completed. In your case 工程热力学代写
Non-electrolyte system
Component 1 __________, component 2 is __________ .
The KNOVEL database (DIPPR 801) can be accessed via our university’s license if you are logged in using VDI connection or locally at the campus
https://app.knovel.com/kn/resources/kpDIPPRPF7/toc?b-q=
Please, search some experimental data sets for the following pure component properties. DIPPR 801 marks if the data point/set is accepted or rejected/not used and put emphasis on the experimental data. Copy the data to Excel so that the information of literature etc. is included.
Select SI-units in Aspen. Gas constant R = 8.31441 Pa*m3/(mol * K)
Your pure component is component ___ . 工程热力学代写
- a) FindtheDIPPR correlation of vapour pressure and some experimental data points. Plot both the correlation and experimental data points into the same graph. How much is the average relative deviation between experimental data and correlation?
- b) Calculatepand dp/dT of DIPPR vapour pressure correlation at T = ____ K.
- c) Findsomeexperimental data of molar density as a function of temperature. Calculate the mass density and molar volume. Program in Excel the Rackett correlation for the molar volume and plot it together with the experimental points. (experimental points are dots and correlation is a continuous line) How much is the average relative deviation between experimental data and Rackett model?
- d) Calculatemolarvolume of the liquid phase at T = ______ K.
- e) Calculatethecompressibility factor based on the virial equation of state or pure component at T = _____ K and its saturated pressure p,sat. Calculate the molar volume of the vapour phase at the same
- f) Calculateenthalpyof vaporization at T = ______ K and find the experimental enthalpy of vaporization close to the T = _____ K to compare the calculated value 工程热力学代写
Binary system
- g) Searchthebinary phase equilibrium data for component 1 ______ and 2 _______ from Aspen “TDE NIST” link. Typically, there is a lot of data available. Run the consistency test for the VLE data and select the most consistent isobaric and/or isothermal data (if available). LLE-data (if available) you need to select visually.
- h) Run the web page http://www.ddbst.com/unifacga.htmlto use “Online Group Assignment for UNIFAC and PSRK”, where input method “DDB Search” of your components. Copythe figure of molecule and its groups into your document.
- i) InAspenselect the UNIFAC-Dortmund (UNIF-DMD). Calculate the phase behavior. This you can do easily in an evaluation mode of regression.
- j) Usingthedrawing tools of Aspen draw the different kind of phase equilibrium data into the same figure if possible. Use merge plot technique. Be careful to select the same component in the horizontal axis. Isothermal VLE and isobaric VLE data are placed on the separate figures. LLE data is merged into the isobaric VLE plot.
- k) Namethe bubble point and dew point curve, non-azeotrope or azeotrope or heterogenousazeotrope, LLE-area, etc.
Electrolyte system 工程热力学代写
Calculation of activity coefficient and solubility for mixed aqueous electrolyte solution
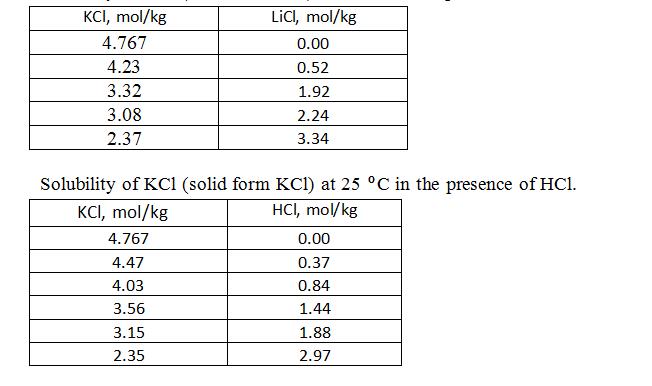
X1) The model electrolytes are KCl – LiCl and KCl – HCl whose SLE data are shown in the following tables reported in literature at 25°C [Solubilities of Inorganic and organic compounds, Volume 3, Ternary and Multicomponent Systems of Inorganic Substances, Part 1, H.L. Silcock (edit.), Pergamon Press, UK, 1979]. KCl is the electrolyte whose solubility is to be determined.
Solubility of KCl (solid form KCl) at 25 ºC in the presence of LiCl.

X2) Calculate the activity coefficient value and further the solubility as molality of the main electrolyte compound in the presence of the given molality of the impurity by using Pitzer model for mixed electrolyte solutions. Submit also the Excel file you used in the calculations.
X3) Plot your obtained KCl solubility as a function of LiCl or HCl molality and the KCl solubility literature data shown in the tables above. Compare the solubility result obtained by Pitzer model to literature data.
Upload the documents into MyCourses. Docx file is the master document, xlsx and apwz files are the supporting information.
Remember to use the naming system of files. 工程热力学代写





发表回复
要发表评论,您必须先登录。